Related Posts
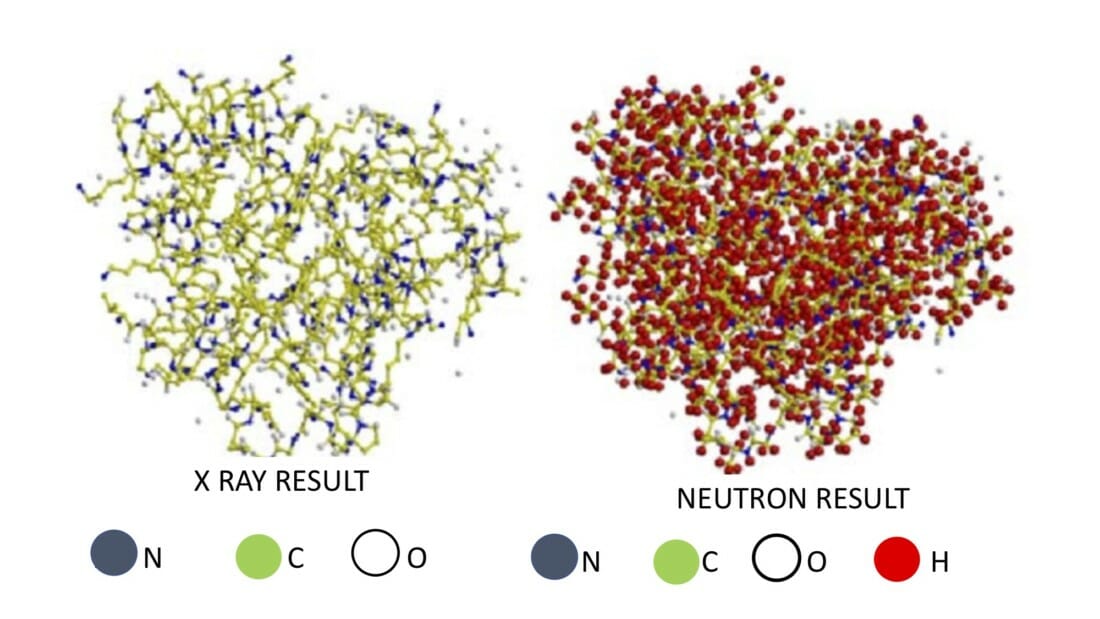
When we examine the world around us, we observe its structure, or where things are, as well as its dynamics, or how things move and interact. Likewise, when we investigate a new material, we want to understand its structure and dynamics—where the atoms and molecules are, and what they are doing. To do this, we need measurement techniques that can tell us what is happening at a very small scale. Read on to find out how neutrons come to our rescue!


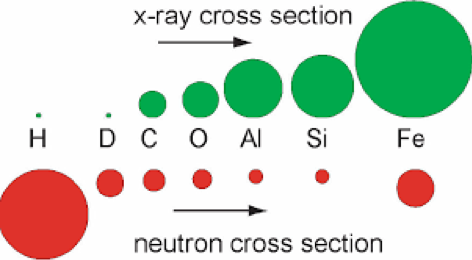
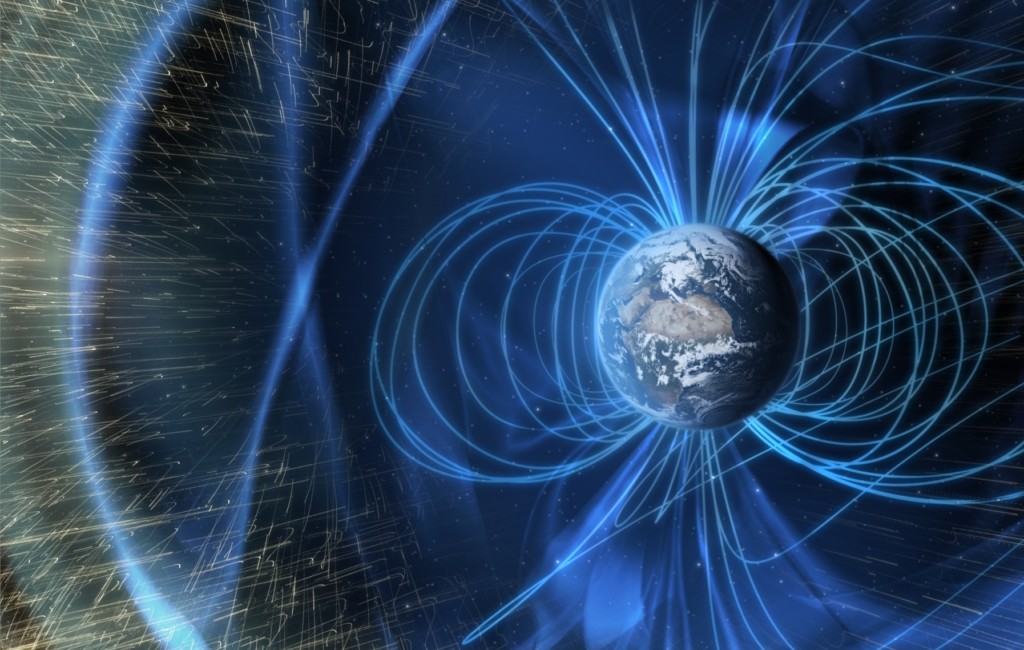
Neutron radiation detection is an important issue for the space program, satellite communications, and national defense. But since neutrons have no electric charge, they can pass through many kinds of solid objects without stopping. This makes it difficult to build devices to detect them, so we need special materials that can absorb neutrons and leave a measurable signature when they do. Researchers at the University of Nebraska-Lincoln are studying the effects of solar neutron radiation on two types of materials on the International Space Station (ISS), using detectors made of very stable compounds that contain boron-10 and lithium-6.


More Funsize Fundamentals
Why do so many fluids behave counterintuitively? Many substances in our lives – like oobleck, slime, or Silly Putty – don’t quite behave the way we expect a fluid to, despite some fluid-like properties. These substances fall into a special category: non-Newtonian fluids. Scientifically, this term is a bit of a catch-all for any substances that have a complicated relationship between their apparent viscosity and the force applied to them.


The term may be unfamiliar, but we all have a sense for viscosity. We often think of it colloquially as the “thickness” of a fluid. It’s the property that makes honey pour so differently from water. Fluid dynamicists – scientists and engineers who study how liquids and gases move – tend to think of viscosity in terms of a fluid’s resistance to flowing or changing its shape.


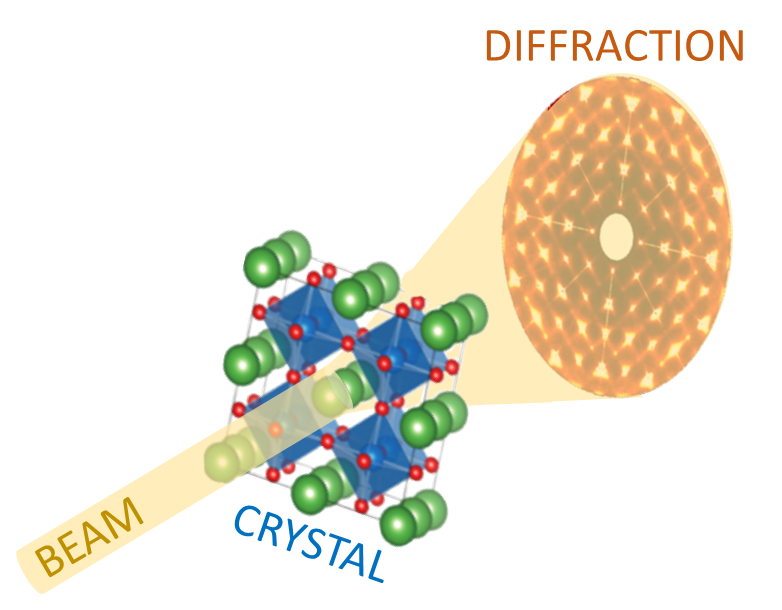
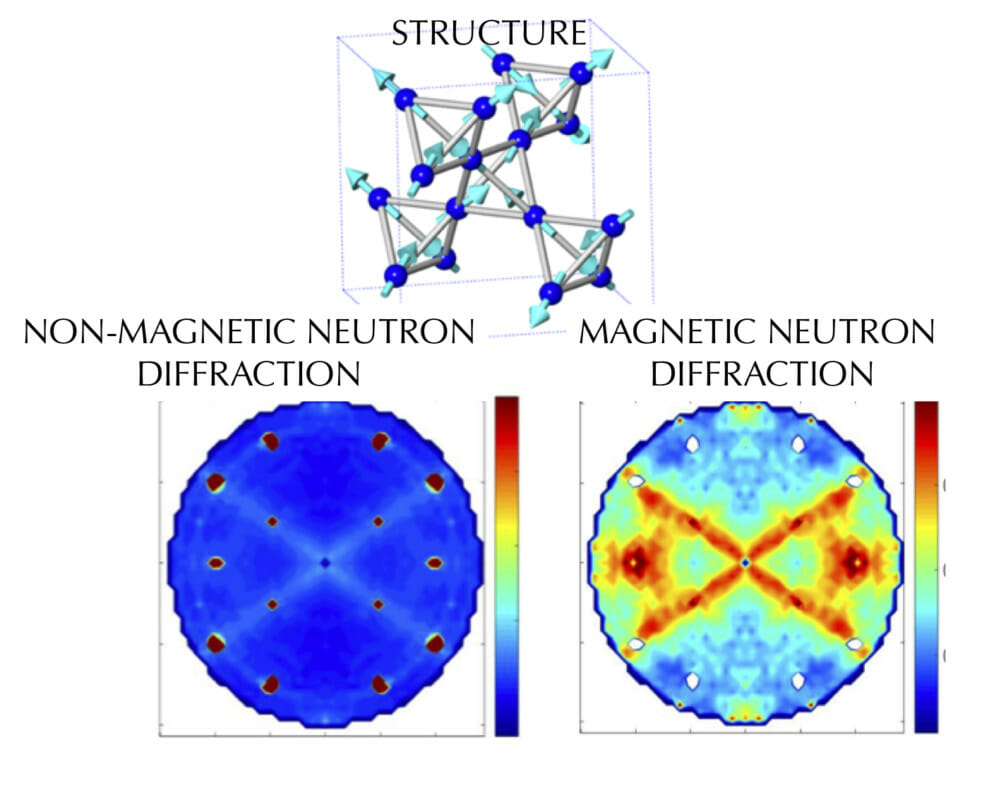
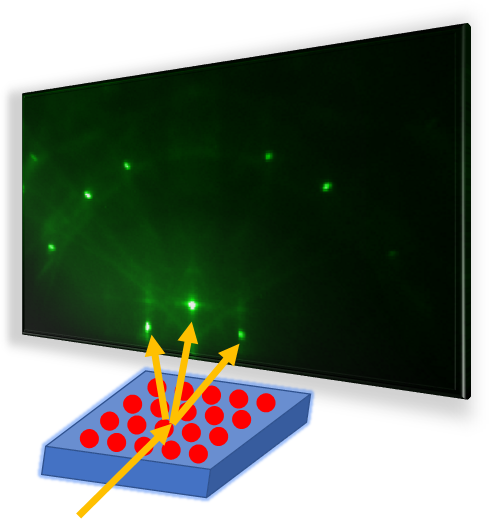
Have you ever wondered why some materials are hard and others soft, some conduct heat or electricity easily while others don't, some are transparent to light while others are opaque . . . and on and on and on? The material universe is vast and diverse, and while a material's properties depend in part on the elements it is made from, its structure—how it is built from its constituent atoms—can also have wide-ranging effects on how it looks, feels, and behaves. Diffraction is a method that allows us to "see" the atomic structure of materials. Read on to find out how it works!


You’re lining up your phone to take a picture of your dog. Light comes down from the sun, bounces off the dog, and into your camera lens, allowing you to take the photo. Your eyes work similarly, taking in all the light particles, known as photons, that are scattering off of objects in the world. Most things “see” by detecting these bouncing photons, which is why both you and your phone have a hard time seeing anything at all when the lights are off.


When light strikes a material, electrons may be ejected from the material. This is called the photoelectric effect, and it’s the basis for many different technologies that convert light energy into electrical energy to generate current. In addition, the photoelectric effect is useful to scientists studying novel materials.