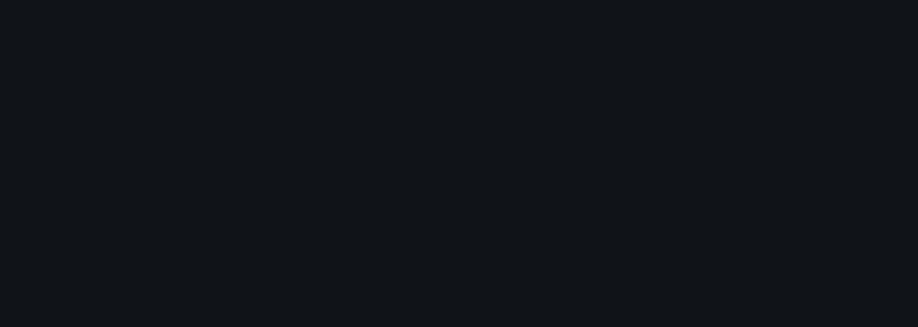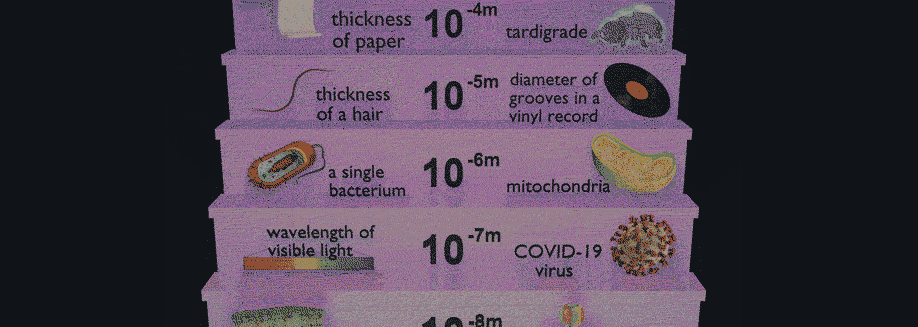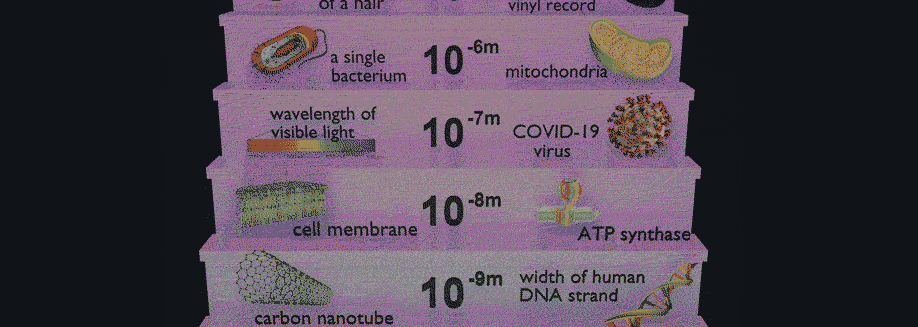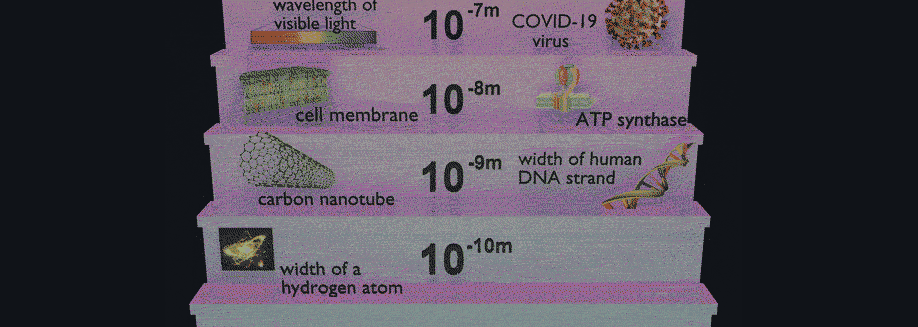
Related Posts
You take a pristine-looking Oreo from a package of seemingly identical sandwich cookies, and you decide to open it up to eat the creme filling first. You gently twist the cookie apart without breaking the chocolate wafers, but the creme sticks to one side only. Why? Happily, the physics of fluids helped two MIT students solve this delicious mystery. Read on to find out what they learned, and how you can test their results at home.


Measurements of the three-dimensional motion of fibers in turbulent fluid flow are helping us understand the multiphase flows involved in making paper.


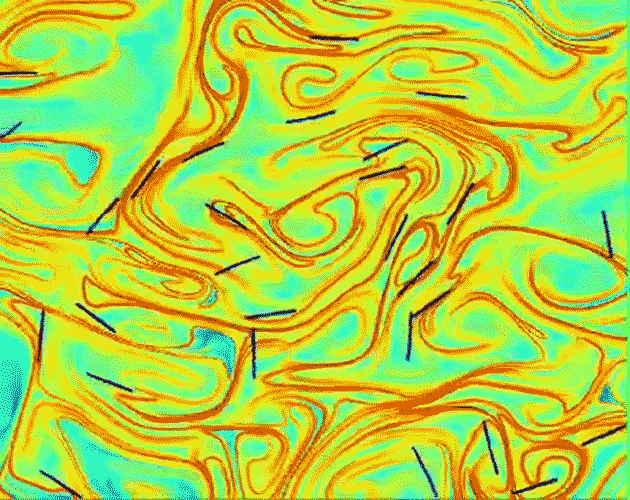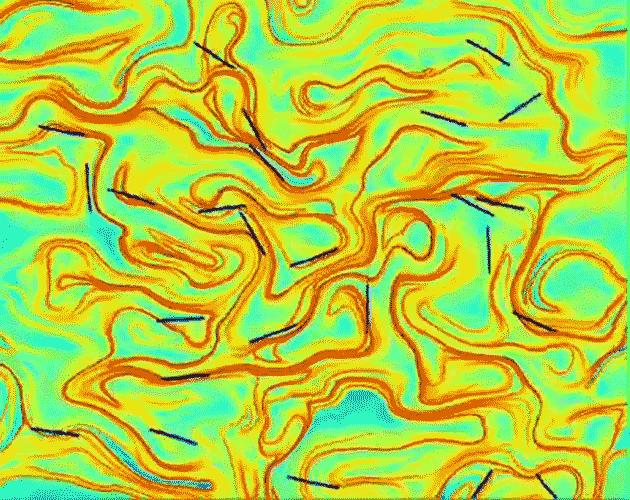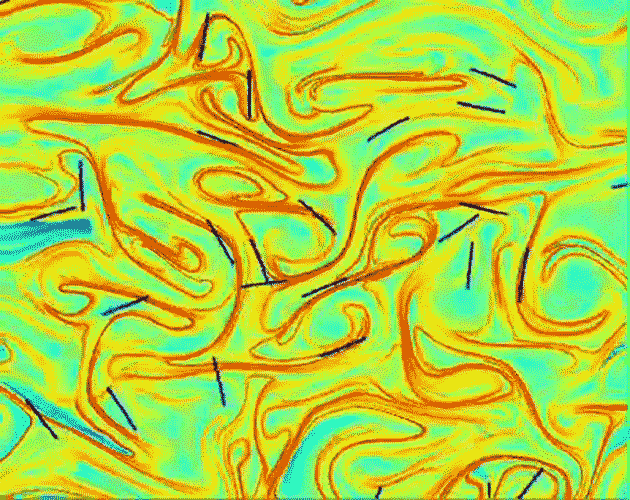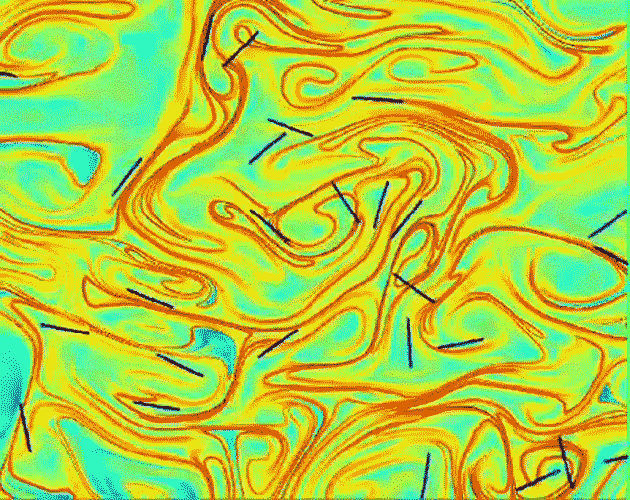
The term may be unfamiliar, but we all have a sense for viscosity. We often think of it colloquially as the “thickness” of a fluid. It’s the property that makes honey pour so differently from water. Fluid dynamicists – scientists and engineers who study how liquids and gases move – tend to think of viscosity in terms of a fluid’s resistance to flowing or changing its shape.


More Funsize Fundamentals
The term may be unfamiliar, but we all have a sense for viscosity. We often think of it colloquially as the “thickness” of a fluid. It’s the property that makes honey pour so differently from water. Fluid dynamicists – scientists and engineers who study how liquids and gases move – tend to think of viscosity in terms of a fluid’s resistance to flowing or changing its shape.


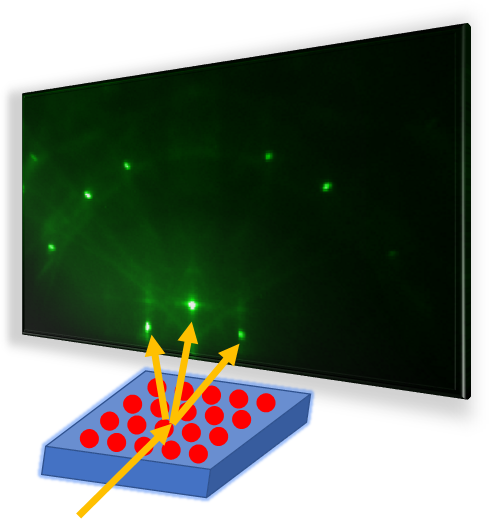
Have you ever wondered why some materials are hard and others soft, some conduct heat or electricity easily while others don't, some are transparent to light while others are opaque . . . and on and on and on? The material universe is vast and diverse, and while a material's properties depend in part on the elements it is made from, its structure—how it is built from its constituent atoms—can also have wide-ranging effects on how it looks, feels, and behaves. Diffraction is a method that allows us to "see" the atomic structure of materials. Read on to find out how it works!


Why do so many fluids behave counterintuitively? Many substances in our lives – like oobleck, slime, or Silly Putty – don’t quite behave the way we expect a fluid to, despite some fluid-like properties. These substances fall into a special category: non-Newtonian fluids. Scientifically, this term is a bit of a catch-all for any substances that have a complicated relationship between their apparent viscosity and the force applied to them.


Surface tension is a somewhat peculiar force. Its effects are all around us, from bubbles and droplets to cleaning our dishes. Surface tension is an important force in our daily lives. But what is it really? Since it tends to act at the scale of millimeters or smaller, we don’t always notice it. It’s critical, however, for many creatures smaller than us, from water-walking insects to star-nosed moles that sniff out food underwater. So what is surface tension and where does it come from?


You’re lining up your phone to take a picture of your dog. Light comes down from the sun, bounces off the dog, and into your camera lens, allowing you to take the photo. Your eyes work similarly, taking in all the light particles, known as photons, that are scattering off of objects in the world. Most things “see” by detecting these bouncing photons, which is why both you and your phone have a hard time seeing anything at all when the lights are off.


When light strikes a material, electrons may be ejected from the material. This is called the photoelectric effect, and it’s the basis for many different technologies that convert light energy into electrical energy to generate current. In addition, the photoelectric effect is useful to scientists studying novel materials.