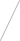ALL POSTS
Real, live scientists sharing cutting-edge research and related classroom activities.
Going With the FFLO
Superconductors and magnetic fields do not usually get along, but a research team led by a Brown University physicist has produced new evidence for an exotic superconducting state that can indeed arise when a superconductor is subject to a strong magnetic field. Their results could enable scientists to develop materials for more efficient memory storage, and even help to explain the behavior of distant astronomical objects called pulsars.


Coloring INSIDE The Lines
Have you ever wondered why shining light on a glass of water causes rainbows to appear? Or noticed the colors that reflect from a CD or DVD? In this lesson, you will make an instrument called a spectroscope that can separate light into its hidden components. You will also be able to use the spectroscope to understand why different colored objects and light sources appear the way they do.


Interacting with the World’s Universal Building Blocks
AtomTouch is a free, interactive molecular simulation app, created by researchers at the University of Wisconsin Materials Research Science and Engineering Center (UW MRSEC) to allow learners to explore principles of thermodynamics and molecular dynamics in an tactile, engaging way.


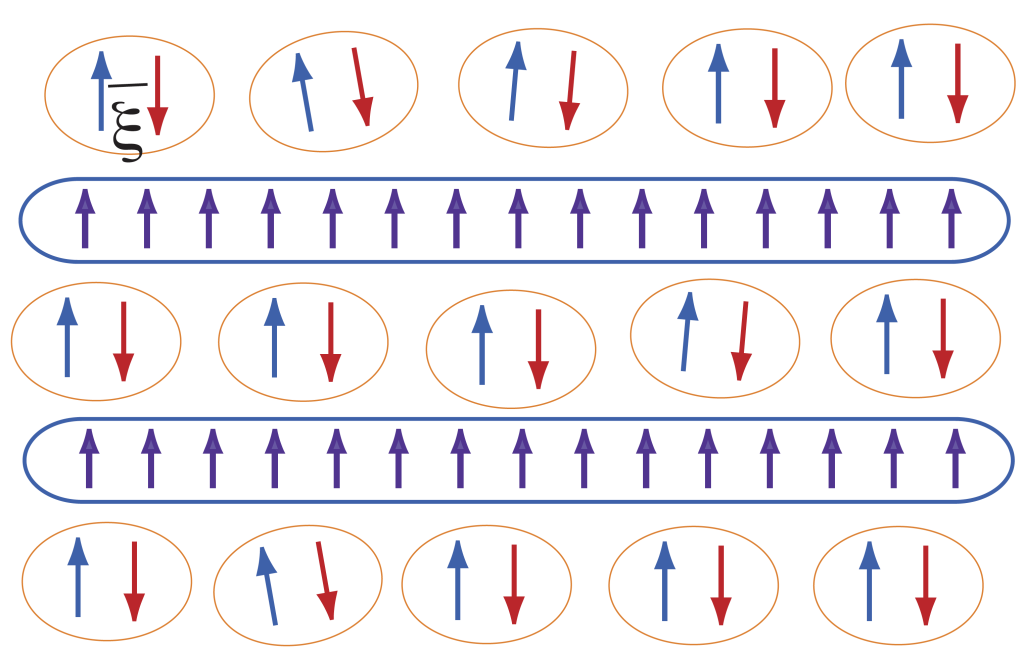
Atomic Legos
Scientists have developed a way to engineer new forms of matter by stacking different types of layers, each only one atom thick, on top of one another.


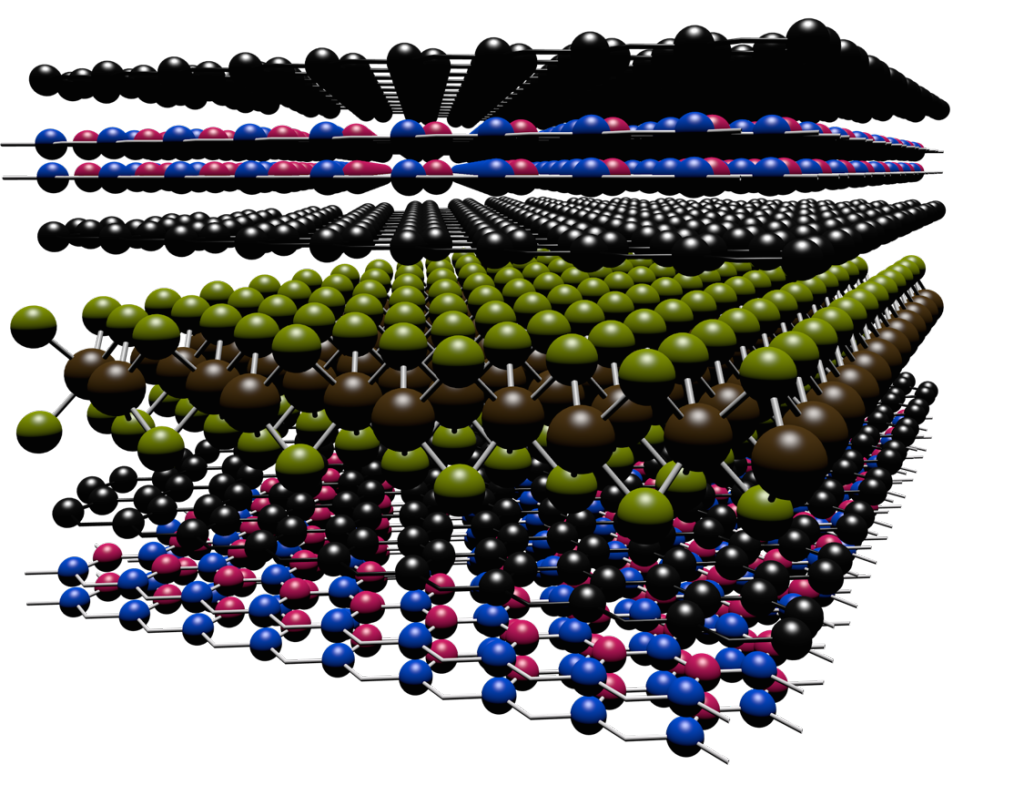
How to Make a Quantum Laser Pointer
Scientists and engineers are making smaller and smaller structures designed to control the quantum states of electrons in a material. By controlling quantum mechanics, we can create new materials that do not exist in nature, develop more efficient solar cells and faster computer chips, and even discover exotic new states of matter.


Dude, where my atoms at?
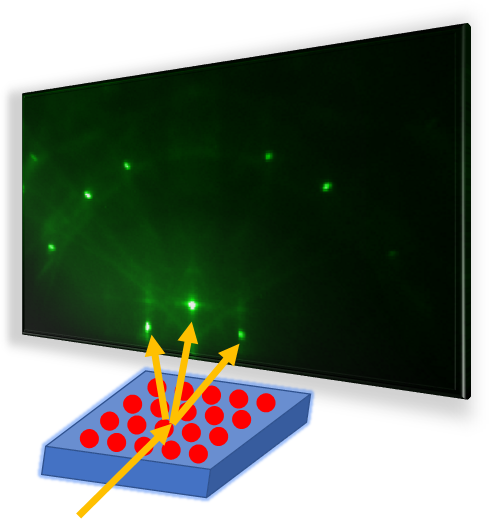
Have you ever wondered why some materials are hard and others soft, some conduct heat or electricity easily while others don't, some are transparent to light while others are opaque . . . and on and on and on? The material universe is vast and diverse, and while a material's properties depend in part on the elements it is made from, its structure—how it is built from its constituent atoms—can also have wide-ranging effects on how it looks, feels, and behaves. Diffraction is a method that allows us to "see" the atomic structure of materials. Read on to find out how it works!


Plot Twist! The Science of Oreology
You take a pristine-looking Oreo from a package of seemingly identical sandwich cookies, and you decide to open it up to eat the creme filling first. You gently twist the cookie apart without breaking the chocolate wafers, but the creme sticks to one side only. Why? Happily, the physics of fluids helped two MIT students solve this delicious mystery. Read on to find out what they learned, and how you can test their results at home.


Neutrons for Nanoscience!
When we examine the world around us, we observe its structure, or where things are, as well as its dynamics, or how things move and interact. Likewise, when we investigate a new material, we want to understand its structure and dynamics—where the atoms and molecules are, and what they are doing. To do this, we need measurement techniques that can tell us what is happening at a very small scale. Read on to find out how neutrons come to our rescue!


Unit Cells and Their Molecular Building Blocks
Through hands-on activities using gumdrops and toothpicks, students will learn about unit cells that make up the repeating structures of crystals like table salt. This lesson is part 2 of a 4-part student-driven, lecture-free series, in which students will do card sorts, build hands-on models, solve engineering design puzzles, and more!


What is Viscosity?
The term may be unfamiliar, but we all have a sense for viscosity. We often think of it colloquially as the “thickness” of a fluid. It’s the property that makes honey pour so differently from water. Fluid dynamicists – scientists and engineers who study how liquids and gases move – tend to think of viscosity in terms of a fluid’s resistance to flowing or changing its shape.


What is a Crystal, Anyway?
Crystals aren't magic, but they are amazing! In this engaging, comic-driven lesson, students do individual and group-based activities to understand the characteristics of crystals (like quartz) versus amorphous solids (like glass). This lesson is part 1 of a 4-part student-driven, lecture-free series in which students will do card sorts, build hands-on models, solve engineering design puzzles, and more!


When you’re small, liquids behave like slippery solids
We think we're pretty familiar with how ordinary liquids behave, but it turns out that some of the basic things we know are no longer true when we look at these liquids on short enough length scales and fast enough time scales. The liquids start to behave more like solids, pushing back when you push on them, and slipping across solid surfaces instead of being dragged along. Click to ride the tiny-but-mighty new wave of nanofluidics!


How do Crystals get their Shapes?
Students make paper models of crystal unit cells and build a large crystal structure together while reflecting on the role of symmetry in crystal formation. This lesson is part 3 of a 4-part student-driven, lecture-free series, in which students will do card sorts, build hands-on models, solve engineering design puzzles, and more!


Ferromagnetic liquids take shape
You may have heard that there are three main phases of matter: solids, liquids, and gases (plus plasma if you want to get fancy). Liquids can take virtually any shape and deform instantly. Solid materials possess interesting electronic and magnetic properties essential to our daily life. But how about designing rigid liquids with magnetic properties? Impossible? Not anymore. Click to learn more!


A Molecular Switch
Think of the hard disk in your computer. Information is stored there in the form of magnetic "bits." But do you know how small a magnet can be? Some molecules make magnetic magic, and these special molecules may give rise to the ultrafast, high precision, low power devices of the future.


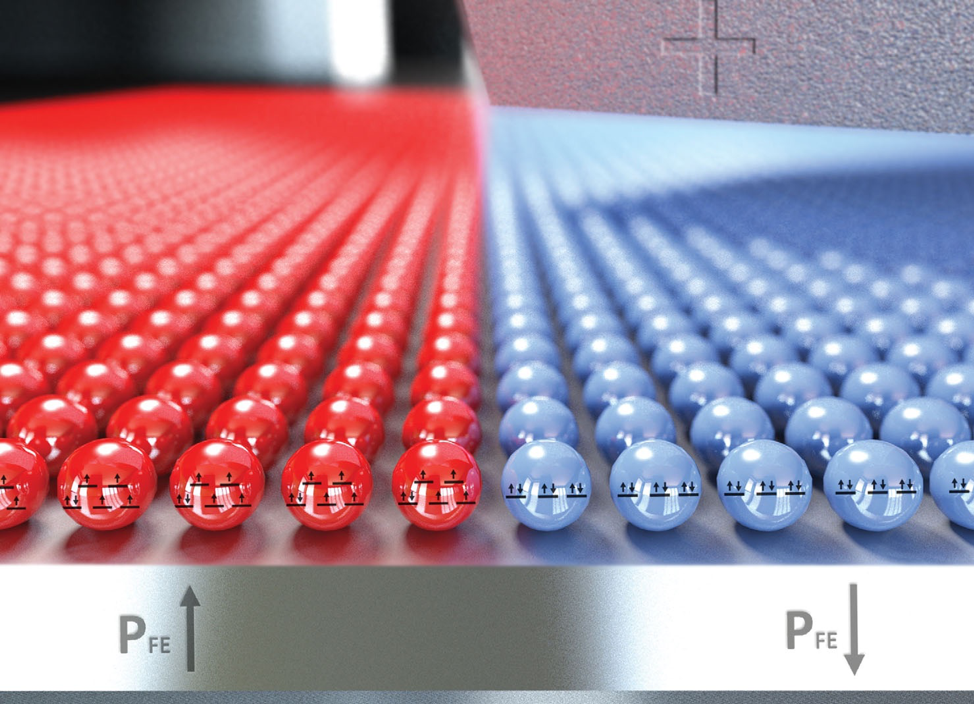
Electric Crystals and their Broken Symmetries
Students learn how some crystals produce electricity when squeezed! This lesson is part 4 of a 4-part student-driven, lecture-free series, in which students will do card sorts, build hands-on models, solve engineering design puzzles, and more!


Magnets, Gatorade, and the Quest for Energy-Efficient Computers
Fool's gold is a beautiful mineral often mistaken for gold, but recent research shows that its scientific value may be great indeed. Using a liquid similar to Gatorade, it can be turned into a magnet at the flick of a switch! Read on to learn more!


The Future is Flat
Welcome to the fascinating world of two-dimensional (2D) materials! Today, we're going to explore a novel 2D material created by boiling off atoms, which we guide to form large crystalline flakes that will become the filling in tiny magnetic sandwiches. Intrigued? Click to learn more!


How Hot Electrons Get Cool
It’s a hot summer day. You desperately want something cold to drink, but unfortunately, your bottle of root beer has been sitting in a hot car all day. You put it into a bucket full of ice to cool it down. But it’s taking forever! How, you wonder, could you speed the process up? The same question is important for understanding how electronic devices work, and how we can make them work better by controlling the temperature of the electrons that power them. Read on to find out what a bottle of root beer in a cooler full of ice and a nanowire in a vat of liquid helium have in common!


Scanning Tunneling Microscopy
You’re lining up your phone to take a picture of your dog. Light comes down from the sun, bounces off the dog, and into your camera lens, allowing you to take the photo. Your eyes work similarly, taking in all the light particles, known as photons, that are scattering off of objects in the world. Most things “see” by detecting these bouncing photons, which is why both you and your phone have a hard time seeing anything at all when the lights are off.